| 6-DIAZO-5-OXO-L-NORLEUCINE Basic information |
| Product Name: | 6-DIAZO-5-OXO-L-NORLEUCINE |
| Synonyms: | 6-diazo-5-oxo,l-norleucin;6-diazo-5-oxo-l-norleucin;diazo-oxo-norleucine;don(pharmaceutical);6-DIAZO-5-OXO-L-2-AMINOHEXANOIC ACID;6-DIAZO-5-OXO-L-NORLEUCINE;6-DIAZO-5-OXO-NORLEUCINE;L-DON |
| CAS: | 157-03-9 |
| MF: | C6H9N3O3 |
| MW: | 171.15 |
| EINECS: | 251-228-4 |
| Product Categories: | Antibiotics |
| Mol File: | 157-03-9.mol |
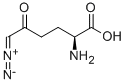
|
|
| 6-DIAZO-5-OXO-L-NORLEUCINE Chemical Properties |
| Melting point | 145 °C |
| Boiling point | 301.12°C (rough estimate) |
| density | 1.3994 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated, Sonicated), Water (Slightly) |
| form | crystalline |
| color | light yellow |
| Merck | 13,3026 |
| BRN | 1725815 |
| Stability: | Hygroscopic |
| InChI | InChI=1/C6H9N3O3/c7-5(6(11)12)2-1-4(10)3-9-8/h3,5H,1-2,7H2,(H,11,12)/t5-/s3 |
| InChIKey | YCWQAMGASJSUIP-GQMHJJTINA-N |
| SMILES | C(C(=O)C= [N+] = [N-] )C [C@H] (N)C(=O)O |&1:7,r| |
| Safety Information |
| Hazard Codes | T |
| Risk Statements | 23/24/25 |
| Safety Statements | 36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | RC6340000 |
| F | 8 |
| 6-DIAZO-5-OXO-L-NORLEUCINE Usage And Synthesis |
| Chemical Properties | Slightly yellow powder |
| Uses | 6-Diazo-5-oxo-L-norleucine (DON) has been used as an inhibitor of glutamine synthetase–glutamine(amide)-2-oxoglutarate aminotransferase. |
| Definition | ChEBI: 6-diazo-5-oxo-L-norleucine is a non-proteinogenic L-alpha-amino acid that is L-norleucine which is substituted at position 5 by an oxo group and at position 6 by a diazo group. It is as inhibitor of various glutamine-utilising enzymes. It has a role as a bacterial metabolite, an analgesic, an antibacterial agent, an antiviral agent, an antineoplastic agent, an EC 6.3.5.5 [carbamoyl-phosphate synthase (glutamine-hydrolysing)] inhibitor, an EC 6.3.4.2 [CTP synthase (glutamine hydrolyzing)] inhibitor, an EC 6.3.5.3 (phosphoribosylformylglycinamidine synthase) inhibitor, an EC 6.3.5.2 [GMP synthase (glutamine-hydrolysing)] inhibitor, an antimetabolite, a glutamine antagonist, an apoptosis inducer, an EC 2.4.2.14 (amidophosphoribosyltransferase) inhibitor, an EC 3.5.1.2 (glutaminase) inhibitor, an EC 6.3.5.1 [NAD(+) synthase (glutamine-hydrolysing)] inhibitor and an EC 6.3.5.4 [asparagine synthase (glutamine-hydrolysing)] inhibitor. It is a non-proteinogenic L-alpha-amino acid, a diazo compound and a ketone. It is a tautomer of a 6-diazo-5-oxo-L-norleucine zwitterion. |
| General Description | Chemical structure: amino acid derivatives |
| Biochem/physiol Actions | DON is used to study mechanisms of glutamine utilizing enzymes such as carbamoyl phosphate synthase and cytidine triphosphate synthase. |
| Purification Methods | Crystallise it from EtOH, H2O/EtOH, MeOH, 95% aqueous MeOH or H2O/Me2CO. [DeWald & Moor J Am Chem Soc 80 3944 1958, Dion et al. J Am Chem Soc 78 3075 1956, Beilstein 4 IV 3278.] |
