|
Description
|
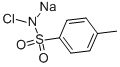
The sodium salt of p-toluenesulfochloramide, commonly known as chloramine-T, exerts strong oxidizing action in both acidic and alkaline media and thus has been widely used for the oxidimetric determination of a large number of inorganic and organic substances. Oxidation of some aldehydes by chloramine-T has been reported to occur quantitatively in an alkaline solution, giving the corresponding acid as the end product. Both direct and indirect methods have been carried out to estimate aldehydes by chloramine-T. Chloramine-T (CAT) is also commonly used in radiolabeling bioactive molecules by halogenation. CAT is used to release radioactive elemental iodine by oxidation of its salts. Unfortunately, CAT is a strong oxidizing agent and can cause significant damage to peptides and proteins. This may lower the yield of the iodination reaction and may produce undesirable side products [1-2] .
|
| Chemical Properties
|
white or yellow powder with a chlorine-like odour
|
|
Uses
|
antiseptic, disinfestant, antiproliferative
|
| Uses
|
This disinfectant is for external use only, it can exterminate bacteria, viruses, fungi, spore. The action principle is that chlorine can sterilize slowly and lastingly, and also can dissolve necrotic tissue, chlorine come from hypochlorous acid which is produced by Chloramine-T solution. Apply to disinfect drinking water container,food,all kind of tableware, fruits and vegetables,and cleaning wound, mucous membrane.
|
|
Uses
|
sterilizer, antiseptic, disinfectant, and chemical reagent in the medical and pharmaceutical fields.
|
| Definition
|
ChEBI: An organic sodium salt derivative of toluene-4-sulfonamide with a chloro substituent in place of an amino hydrogen.
|
|
Flammability and Explosibility
|
Not classified
|
| Safety Profile
|
Poison by parenteral and intravenous routes. Human mutagenic data reported. When heated to decomposition it emits toxic fumes of Cl-, SOx, Na2O, and NOx. See also SULFONATES and CHLORIDES.
|
|
Synthesis
|
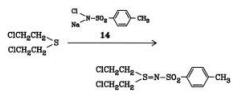
Chloramine-T is prepared in 75 – 95 % yield by passing chlorine into a sodium hydroxide solution of p-toluenesulfonamide. It is a strong electrolyte in acid solution and a good oxidizing agent in base. It is fairly soluble in water, and practically insoluble in benzene, chloroform, and ether. The compound reacts readily with mustard gas to yield a harmless crystalline sulfimide; chloramine-T derivatives are being studied as protective agents against poison gas.
|
| References
|
[1] M. C. Agrawal, S. P. Mushran. "Mechanism of Oxidation of some Aliphatic Aldehydes by Chloramine-T." Zeitschrift für Naturforschung B 20 1 (1972): 401–404.
[2] B M Tashtoush. "Chloramine-T in radiolabeling techniques. IV. Penta-O-acetyl-N-chloro-N-methylglucamine as an oxidizing agent in radiolabelling techniques." Analytical biochemistry 288 1 (2001): 16–21.
|